- Fruit
- Edible Oil
- Stuffed Pasta
- Crops Grains Oilseeds and Rice
- Grain Milling and Malt
- Vegetable Dried Beans
- Natural Plant Spices
- Nuts and Seeds
- Dried Fruits
- Unroasted coffee&cocoa beans
- Special Dietary Foods
- Functional foods
- Eggs and egg products
- Meat and meat products
- Aquatic products
- Aquatic Animals
- Dairy
- Casings
- Bird's Nest
- Bee products
- GMO
- Non-edible animal products
- Chinese herbal medicine
- General Foods
- GACC Evaluates CA
- Cotton Supplier Registration
Functional foods
Health food refers to foods that claim to have specific health functions or for the purpose of supplementing vitamins and minerals, that is, suitable for consumption by specific groups of people, regulating body functions, not for the purpose of treating diseases, and do not cause any acute, subacute or chronic harm to the human body food.
"Health food" are generally called in mainland China, and other countries or regions are called: Dietary Supplements. In Europe and the United States, it is called "nutritional food".It is included in the category of "specific nutritional food". If a health food category is exported to China for the first time, GACC must first conduct a conformity assessment on the official regulatory system of the country of origin.
GACC registration conditions for overseas manufacturers of imported foods are as follows:
1. The food safety management system of the country/region where the manufacturer is located has passed GACC's equivalence assessment and/or review;
2. The manufacturer was established with approval by the competent authority of the country/region, and the manufacturer is under effective regulation by the competent authority;
3. The manufacturer has an established, effective food safety and sanitation management system and protection system, legally produces and exports food in the country/region, and ensures that foods exported to China comply with relevant Chinese laws, regulations, and national food safety standards;
4. Food exporting to China conforms with relevant inspection and quarantine requirements that have been agreed upon after discussion by the GACC and the competent authorities of the country/region.
The Chinese national standards and regulations that GACC is required to comply with include but are not limited to:
1. National Food Safety Standard Good manufacture practice for Health Food GB17405-1998
2. National Food Safety Standard Health Food GB 16740-2014
3. National Standard for Food Safety General Rules for Labeling of Prepackaged Foods GB 7718-2011
4. National Standard for Food Safety Food Additive Use Standard GB 2760-2014
5. National Food Safety Standard General Hygienic Specification for Food Production GB 14881-2013
6. Food defense plan and its application guide for food production enterprises GB T 27320-2010
7. Hazard Analysis and Critical Control Points (HACCP) System General Requirements for Food Manufacturers GB/T 27341
(If you need to obtain the English version of the above regulations or technical standards, or the official language version of your country, feel free to contact us)
• GACC registration process (text description)
• The flow chart for the GACC registration of imported food of overseas producers
• Registration conditions and essential checkpoints for functional food
The General Administration of Chinese Customs, overseas official regulatory agencies and production enterprises will jointly promote:
-
01
Confirm the HS code and the applicable access method.
-
02
Obtain the account and password officially granted by the supervisor or account re-authentication.
-
03
Prepare documents in strict accordance with Conditions and Key Points of Control Inspection for Registration of Overseas Manufacturers of Imported Health Food, conduct self-assessment, and fill in the registration application.
-
04
Submit an electronic application, fill in the enterprise elements and upload relevant materials as required; if necessary, include but not limited to floor plan of factory and workshop, process flow chart, etc.
-
05
The competent authority reviews the summary and forwards the electronic application with other support materials required by GACC.
-
06
The GACC office will review whether the materials are complete and in compliance with the legal form, and decide whether to accept it or issue a correction notice to the competent authority.
-
07
The expert group evaluates and reviews, including a combination of document review and on-site review or other reasonable review methods, and forms a review report.
-
08
Review by the General Administration of Chinese Customs whether it meets the statutory requirements and standards.
-
09
GACC approve or disapprove the registration, and feedback the information to the competent authority and Enterprise (enterprise applicants can receive the registration feedback in Cifer system).
-
10
The enterprises approved for registration, China Customs will publish them online in due course.
Our assistance includes but is not limited to:
1. Assist in product classification, determine the corresponding registration method and the best implementation plan; confirm the applicable official competent authority approval number, enterprise type, precise customs code and inspection and quarantine code details.
2. Assist enterprises to obtain account numbers and passwords.
3. Assist manufacturer to review the registration elements according to the "Registration conditions and essential checkpoints for functional food", complete the self-assessment, and provide continuous improvement solution based on the assessment results, so that they meet the registration conditions and check points and China's national food production and product standards. Assist in sorting, screening, editing, and translating the required materials, so that the application materials meet the integrity, authenticity and validity.
4. Assist to fill in the Registration Application of Overseas Health Food Imported Manufactures Recommended by Competent Authority and submit the application.
5. Follow up the application submitted by the competent authority, the basic status report on animal and plant epidemics, veterinary hygiene, public health, plant protection, pesticide and veterinary drug residues related to the exporting country (region), the registration management of food production enterprises and the requirements of enterprise hygiene standards, etc. The laws, regulations and standards of the exporting country (region), the organization and personnel of the competent authority of the exporting country (region), the quarantine of the exporting country (region) competent authority of its recommended enterprises, the assessment answer sheet of the actual situation of sanitation control, the exporting country (regional) competent authority's assessment of the actual situation. A statement that the companies that are recommends comply with Chinese laws and regulations.
6. Materials correction, when the written materials do not meet the registration requirements, assist the enterprise to complete the interpretation, sorting, screening, editing, translation, calibration and other standards and laws and regulations of the required materials within the specified time.
7. Acceptance by Chinese Customs and follow-up and communication of technical review by expert group, preparation and coordination before on-site review or video review.
8. Assist in successful access and follow up on the use of registration data for customs clearance, and respond immediately if there are any problems.
9. According to the change of the enterprise, assist the change application.
10. Other enterprise access and follow-up related support.
It refers to foods that claim to have specific health-care functions or for the purpose of supplementing vitamins and minerals, that is, foods that are suitable for specific population to eat, capable of regulating body functions, not for the purpose of treating diseases, without causing any acute, subacute or chronic harm to human body.
With "Health-care Product" as the general name in mainland China, they are generally called as "Dietary Supplements" in other countries or regions. In Europe and the United States, they are called as "Health-Care Food" or "Health Food", also known as "Nutrition Food"; In Germany, they are called as "Improved Food"; In Japan, they are called as "Functional Food" or "Foods for Specified Health Use", and included in the category of "Specific Nutrition Food" .
Regulatory Measures:
1. Registration of overseas production enterprises
2. Record-keeping for overseas exporters
3. Record-keeping for Chinese importers
4. Approval documents for Health-care food (for registration or record-keeping)
Solutions for meet requirements of Regulatory Measures:
1. Registration of overseas manufacturing enterprises
Solution: Register through the cifer system of Customs. After receiving the application recommended by overseas officials, the GACC shall form an expert panel to review and grant the registration number for exporting to China.
2. Record-keeping for overseas exporters
Solution: Submit the application through “Internet + Customs” - “Record System for Importers and Exporters of Imported Food”.
3、Record-keeping for Chinese importers
Solution: Submit the application through “Internet + Customs” - “Record System for Importers and Exporters of Imported Food”.
4. Approval documents for Health-care food (for registration or record-keeping)
Record-keeping for imported health-card food:
1. For imported health-care foods that supplement vitamins and minerals, please refer to the Catalogue of Raw Materials for Health-Care Food -Nutrient Supplement.
2. Subject to the constraints of excipients, dosage forms, health-care functions and dosage.
3. Subject to approval by the State Administration for Market Regulation.
Process of record-keeping for imported health-card food:
Provide samples for filing inspection -- sort out filing information -- apply for user name and password of filing information system -- fill in the filing information system -- submit filing information -- pass the interview and obtain the filing voucher
Application materials for record-keeping of imported health-care food (depending on different conditions of products):
1. Registration Form for Record-keeping of Imported Health-care Food, as well as the Statement for Undertaking Legal Liability of the filing person for being responsible for the authenticity of the submitted materials
2. A copy of the registration certificate of the subject of filing person
3. Product formula materials: Product Formula Table
(In case of any change in the formula, the original registrant shall submit the adjusted formula and description of the formula change)
4. Production process materials, including the diagram and description of the production process
5. Materials for evaluation of safety and health care functions
5.1 Test reports on efficacy ingredients or iconic ingredients, hygiene and stability of three batches of products produced by pilot-scale or above processes
(This is not required if the original registrant's product formula has not changed)
5.2 Instructions on the rational use of raw materials and excipients, as well as the statement that the formulation of label instructions and product technical requirements are in accordance with relevant laws and regulations
6. Types, names and relevant standards of packaging materials directly in contact with health-care food
7. Sample draft of label specification of the product
8. Product technical requirement materials
9. Full-item test report conforming to the technical requirements of the product issued by an testing agency with legal qualifications
9.1 CMAF Qualification certification documents
9.2 Full-item test report of three batches conforming to product technical requirements
10. Product name related Retrieve materials
11. Other materials that indicate the product security and health-care functions
12. The qualification certification documents issued by the competent government authority or legal service agency of the manufacturing country (region) of the product proofing that the filing person is a listed overseas manufacturer of health-care food
13. Certificate of health-care food products marketed for more than one year issued by the competent government authority or legal service agency of the manufacturing country (region) of the product, or safety report on the overseas sales of the product and the consumption of the population
14. Original text of technical regulations or standards related to health-care food of the manufacturing country (region) or international organization
15. Real samples of packages, labels, instructions, Chinese translation and notarial certificate of the product listed in the manufacturing country (region)
16. Where the filing affairs are handled by the resident representative office of the foreign filing person in China, the registration certificate of the resident representative office of the foreign filing person in China and its copy shall be provided. Where the overseas filing person entrusts a domestic agency to handle the filing affairs, it shall submit the original notarized power of attorney and a copy of the business license of the entrusted agency.
Registration of Imported Health-care Food:
1. Imported health food (health-care food raw materials except supplements of vitamins and minerals).
2. Subject to approval by the State Administration for Market Regulation.
The application for health-care food registration is highly professional and takes a long time to review (usually 5-10 years). It has comprehensive requirements for product formula, preparation technics, method demonstration, equivalence study, test report and clinical data. Please contact us for the best registration solution according to the specific situation of the product.
Health food labels and instructions must comply with relevant national standards and requirements, and indicate the following:
(I) Health-care function and suitable population;
(II) Consumption method and dosage;
(III) Storage method;
(IV) Names and contents of efficacy ingredients;
(V) Approval number of health-care food;
(VI) Mark of health-care food;
(VII) Other label contents specified by relevant standards or requirements;
Additional notes:
(I) The names of health-care food should be accurate and scientific, no person name, place name, code, or exaggerated names that are easy to be misunderstood shall be used.
(II) The content of the labels, instructions and advertisements of health-care food must be true and conform to the quality requirements of the product, and there must be no propaganda suggesting that it can cure any disease.
Information required for Customs declaration:
1. Certificate of Origin
2. Packing List
3. Invoice
4. Contract
5. Bill of Lading
6. Sanitary certificate (certificate of free sale or health certificate)
7. Test report
8. List of ingredients
9. Application form for import of health-care food
10. Formula, production process and quality standard of health-care food
11. Toxicological safety evaluation report
12. Health function evaluation report
13. List of functional ingredients of health-care food, qualitative or quantitative test methods and stability test report of efficacy ingredients
14. Sample of the product and its hygiene inspection report
15. Labels and instructions
16. Letter of authorization for use of trademark or processing
17. Other supporting information or certificate
Imported health food with the following customs codes is monitored under Decree No. 248:
| HS Codes | Prodcut Name | CIQ Codes | Inspection and quarantine name | Product Category |
| 1504100010 | Endangered fish liver oil and its isolates | 101 | Endangered fish cod liver oil and its isolates (edible health food) | Healthy food |
| 1504100090 | Other fish liver oil and its fractions | 201 | Other fish liver oil and its separated products (health food) | Healthy food |
| 1504200011 | Endangered fish oil soft capsules | 101 | Endangered fish oil soft capsules (except cod liver oil) (health food) | Healthy food |
| 1504200019 | Endangered fish other fish oils, fats and their fractions | 107 | Endangered fish other fish oils, fats and their fractions (except cod liver oil) (health food) | Healthy food |
| 1504200091 | Other fish oil soft capsules | 101 | Other fish oil soft capsules (except cod liver oil) (health food) | Healthy food |
| 1504200099 | Other fish oils, fats and their fractions | 107 | Other fish oils, fats and their fractions (except cod liver oil) (health food) | Healthy food |
| 2104200000 | Homogenized mixed food | 104 | Homogenized mixed food (with health food approval number) | Healthy food |
| 2106100000 | Concentrated protein and artificial protein material | 102 | Concentrated protein and artificial protein substances (with health food approval number) | Healthy food |
| 2106903010 | Royal jelly formulation containing endangered plant components | 101 | Royal jelly preparation containing endangered plant ingredients (with health food approval number) | Healthy food |
| 2106903090 | Other royal jelly preparations | 101 | Other royal jelly preparations (with health food approval number) | Healthy food |
| 2106905010 | Endangered Seal Oil Capsules | 999 | Endangered seal oil capsules (edible health food) | Healthy food |
| 2106905090 | Other seal oil capsules | 999 | Other seal oil capsules (edible health food) | Healthy food |
| 2106909019 | Other numbered unlisted foods containing endangered animal and plant ingredients | 107 | Other numbered unlisted foods containing endangered animal and plant ingredients (with health food approval number) | Healthy food |
| 2106909090 | Food not listed in other numbers | 140 | Other foods whose numbers are not listed (with health food approval number) | Healthy food |
| 2202100010 | Flavored, sweetened or other sweetened water containing endangered animal and plant ingredients (including mineral water and soft drinks) | 108 | Flavored, sweetened or other sweetened water containing endangered animal and plant ingredients (including mineral water and soft drinks) (with health food approval number) | Healthy food |
| 2202100090 | Other flavored, sweetened or other sweetened water (including mineral water and soda) | 108 | Other flavored, sweetened or other sweetened water (including mineral water and soft drinks) (with health food approval number) | Healthy food |
| 2202990011 | Other bulk non-alcoholic beverages containing endangered animal and plant ingredients (excluding fruit juice or vegetable juice of item 20.09) | 108 | Other bulk non-alcoholic beverages containing endangered animal and plant ingredients (excluding fruit juice or vegetable juice of item 20.09) (with health food approval number) | Healthy food |
| 2202990019 | Other bulk non-alcoholic beverages (excluding fruit or vegetable juices of item 20.09) | 999 | Other bulk non-alcoholic beverages (excluding fruit juice or vegetable juice of heading 2009) (health food) | Healthy food |
| 2202990091 | Other packaged non-alcoholic beverages containing endangered animal and plant ingredients (excluding fruit juice or vegetable juice of item 20.09) | 108 | Other non-alcoholic beverages in other packages containing endangered animal and plant ingredients (excluding fruit juice or vegetable juice of item 20.09) (with health food approval number) | Healthy food |
| 2208909021 | Potato distilled liquor containing endangered wild animals and plants | 105 | Potato distilled spirits containing endangered wild animals and plants (with health food approval number) | Healthy food |
| 2208909091 | Other distilled spirits and alcoholic beverages containing endangered wild animals and plants | 106 | Other distilled spirits and alcoholic beverages containing endangered wild animals and plants (with health food approval number) | Healthy food |
| 2209000000 | Vinegar and substitutes for vinegar made with acetic acid | 104 | Vinegar and vinegar substitutes made with acetic acid (with health food approval number) | Healthy food |
| 2936280000 | Unmixed vitamin E and its derivatives | 102 | Unmixed vitamin E and its derivatives (whether soluble in solvent or not) (with health food approval number) | Healthy food |
| 2936901000 | Vitamin AD3 | 102 | Vitamin AD3 (including natural concentrates, whether soluble in solvents or not) (with health food approval number) | Healthy food |
| 2936909000 | Provitamins, mixed provitamins, other mixed vitamins and their derivatives (including natural concentrates, whether or not dissolved in solvents) | 102 | Provitamins, mixed provitamins, other mixed vitamins and their derivatives (including natural concentrates, whether soluble in solvents or not) (with health food approval number) | Healthy food |
| 3004905110 | Chinese medicinal wine containing endangered animal and plant ingredients (already prescribed dose or retail packaging) | 102 | Chinese medicinal wine containing endangered animal and plant ingredients (prescribed dose or retail packaging) (with health food approval number (health food)) | Healthy food |
| 3004905190 | Chinese medicinal wine containing other ingredients (already prescribed dose or retail packaging) | 102 | Chinese medicinal wine containing other ingredients (already prescribed dose or retail packaging) (with health food approval number) | Healthy food |
| 3504001000 | Peptone | 101 | Peptone (with health food approval number) | Healthy food |
| 3504009000 | Other proteins and their derivatives (including peptone derivatives and skin powder (whether or not chrome alum is added)) | 101 | Other proteins and their derivatives (including peptone derivatives and skin powder (whether or not added with chrome alum)) (with health food approval number) | Healthy food |
| 7116100000 | Natural or cultured pearl products | 101 | Natural or cultured pearl products (with health food approval number) | Healthy food |
1. What are the common mistakes in registration?
(1) The product was classified incorrectly, and the wrong access method and procedure were applied.
(2) Failure to strictly follow the "Registration conditions and essential checkpoints for functional food" to inspect the elements of the enterprise and conduct feasibility assessments.
(3) Electronic application has not been submitted. "Application for Registration of Overseas Officially Recommended Manufacturers of Imported Health Foods"
(4) Registration application materials or attachments submitted are not in English or Chinese.
(5) The file format is incorrect and cannot be opened.
(6) There is a vacancy in the application materials where the signature and seal of the competent authority is required.
(7) The necessary parts of the application form are incomplete.
(8) The attachments of the application form are incomplete or do not correspond to the description of the content of the application form, which cannot prove the validity of the content of the application form.
(9) There are descriptions in the application form and attached materials that are obviously inconsistent with Chinese regulations.
(10) The information of the exported products cannot be accurately listed in the application, so that the customs clearance failure and needs to be urgently added. (Overseas manufacturers generally register according to the actual product strictly corresponding to the HS CODE, but Chinese brokers or freight agencies generally classify HS CODE according to the most preferential tax rate or the lowest supervision conditions or the simplest operation methods. There are cases that different ports and different freight agencies use different HS CODE for the same foods. There is a possibility of different classification, and our agency have to deal with many emergencies caused by the reasonable but misplaced demands of different market entities.)
(11) The application materials provided are contradictory.
(12) The country/origin where the overseas producers is located export firstly some foods has not yet conducted conformity equivalent assessment of the GACC official regulatory system.
2. An example of GACC does not approve review feedback?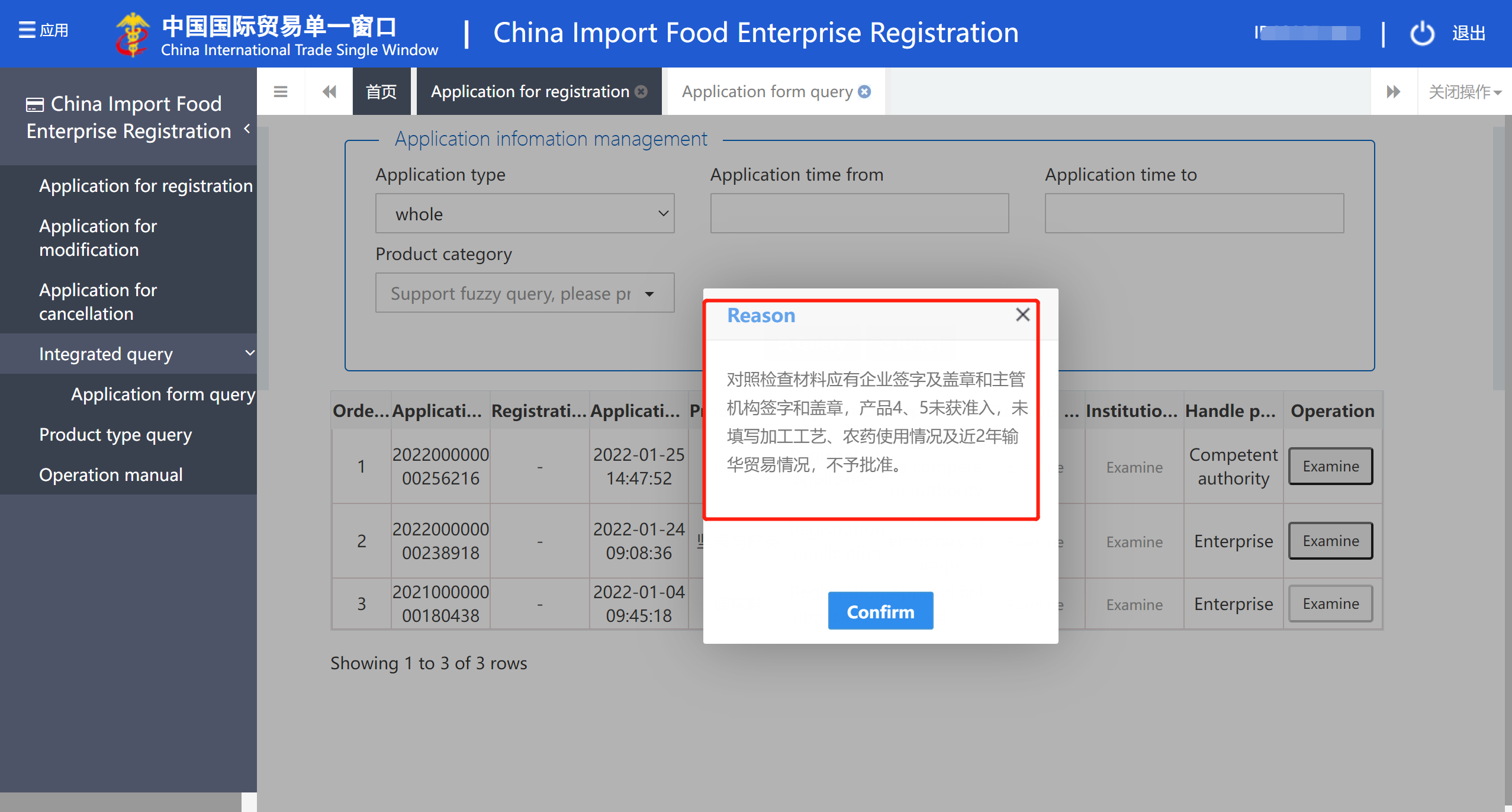
3. What's the labeling requirements of imported health food?
Answer: According to Decree No.249 Article 30:The packagings, labels, and marks of import food shall comply with the laws, regulations, national food safety standards of China, and shall be accompanied by an instruction manual in Chinese if the law so requires.The label of an imported dietary supplement or food for special dietary use in Chinese must be printed on the smallest sales package, and shall not be affixed thereto
4. Do overseas manufacturers, exporters and domestic importers need to register for the cross-border e-commerce model?
Answer: According to the "Notice of Improving the Supervision of Cross-border E-commerce Retail Imports" and the "List of Cross-border E-commerce Retail Import Commodities", it is limited to personal use and meets the requirements of cross-border e-commerce. For goods imported from overseas e-commerce retail, the bonded stocking mode "first-line" entry area is regulated according to the goods, and the above-mentioned domestic and overseas enterprises need to register or record with GACC.
5. Are cross-border e-commerce exempt from the regulatory conditions for imported health food?
Answer: According to the "white list" system, the cross-border e-commerce model is exempt from the regulatory conditions for imported health food, that is, the product does not need to be filed or registered, and does not need Chinese labels.
6. Can the so-called "health food" be imported as ordinary food?
Answer: Yes.It can be declared according to ordinary food, but there must be national standards applicable to ordinary food. It is either imported according to the new resource food or according to the approval document of health care products. These two difficulties are similar and then the problem of dosage form. If there is no special publicity on the outer packaging of applicable national standard products, then even special dosage forms can be imported, such as It is said that effervescent tablet is a tablet, and coffee is a granule, all of which can be imported.
7. What kind of health functions must be registered?
Answer: In addition to the prescribed filing, other imported products that claim to have health care functions must be registered. The 27 functions are: (1) Strengthening power; (2) Auxiliary lipids; (3) Auxiliary sugars; (4) Antioxidation; (5) ) to help improve memory; (6) to relieve visual fatigue; (7) to clear the throat; (8) to assist in reducing blood pressure; (9) to promote lead excretion; (10) to improve sleep; (11) to promote lactation; (12) to relieve physical fatigue (13) Improve hypoxia tolerance; (14) Have auxiliary protective function against radiation hazards; (15) Lose weight; (16) Improve growth and development; (17) Increase bone density; (18) Improve nutritional anemia; ( 19) Auxiliary protection against chemical liver damage; (20) Acne removal; (21) Melasma removal; (22) Skin moisture improvement; (23) Skin oil improvement; (24) Intestinal flora regulation ; (25) promote digestion; (26) laxative; (27) have auxiliary protective function on gastric mucosal damage.
8. What is the validity period of the health food filing certificate or registration approval document?
Answer: The imported health food filing certificate has no time limit and can be used for a long time without renewal. The registration approval for imported health food is valid for 5 years, and re-registration (ie.renewal) is required upon expiration.
9. Do exporters or shippers need to record?
A: Yes, the exporter of the trade contract or shipper of BL has to do ire (Original AQSIQ) record. After recording will get an 11-digit record number, the record does not issue certificate nor validity limit. It should be noted that this record is very easy, we are obliged to help applicant record free of charge. Watch out for fraudulent websites.
(In China's administrative reform, AQSIQ no longer exist)
10. What are the health functions claimed by nutrient supplements allowed in health foods?
A: For details, please refer to the "List of Health Functions Permitted to Claim Health Foods Nutrient Supplements"
11. What are the dosage forms and technical requirements for health food filing products?
A: For details, please refer to the "Dosage Forms and Technical Requirements of Health Food Filing Products"
12. What are the excipients available for health food filing products and their use regulations?
A: For details, please refer to the Regulations on the Provisions on the Use of Excipients and Their Use in Health Food Recordation Products
13. What are the raw materials available for registration of health food produced overseas?
A: For details, please refer to the Guidelines for Raw Materials Available for Registration of Health Food Produced Overseas (Applicable Registration)
14. What are the raw materials available for the recordation of overseas production of health food?
A: For details, please refer to the Guidelines on Raw Materials Available for the Filing of Health Food Produced Overseas (Applicable Filing)
15. What are the raw materials available for the registration of health food produced in China?
A: For details, please refer to the Guidelines for the Registration of Raw Materials Available for the Registration of Health Food Produced in China (Applicable Registration)
16. What are the raw materials available for the recordation of health food produced in China?
A: The "Health Food Raw Material Catalogue Nutrient Supplements" (i.e. the list of vitamins and minerals) and the five nutrients of coenzyme Q10, spirulina, melatonin, broken wall Ganoderma lucidum spore powder, and fish oil can be recorded.
17. Do raw materials produced overseas for the production of health food need to be registered or recorded?
A: Most ordinary food raw materials can be directly produced overseas without prior trade conditions, and the products can meet Chinese national standards. Part of this depends on the access status of countries that meet the requirements of the assessment review and have traditional trade. Chinese medicinal materials depend on the country and specific product access. New resource food depends on the approval announcement, etc. All in all, the catalogue, category and attributes of health food raw materials are different, and specific products should be determined on a case-by-case basis, while consciously paying attention to relevant agreements, protocols and inspection and quarantine requirements.
18. Do products produced with formulas developed with available raw materials for health food have to be registered?
A: No. Look at the specific raw materials and the claims of health care functions, such as the varieties of Chinese medicinal materials for both medicine and food, ordinary food raw materials and new food raw materials. Another reminder is that it can be registered, does not mean that registration can be performed, such as chaga, cinnamomea cinnamomea, enzyme and a few other although in the health food registration raw material catalog, but lack of Chinese safety data support will not be approved, it is recommended not to register.
Related Laws and Regulation of Imported Health Food are including but not limited to:
Measures for the Administration of the Registration and Recordation of Dietary Supplements
Health Food Record Filing Guidelines (Trial)
Health Food Raw Material Catalog Nutrient Supplements (2020 Version)
List of Health Functions Allowed for Health Food Claims Nutrient Supplements (2020 Version)
Health food Record filing product dosage form and technical requirements (2021 Version)
Coenzyme Q10 and other five health food raw materials record product dosage form and technical requirements 2021[NO.4]
Complementary Interpretation of "Formulations and Technical Requirements for Recorded Products of Five Health Food Raw Materials including Coenzyme Q10"
Health food products available for record excipients and their use regulations(2021 Version)
Q10 Health Food Raw Material Catalog -Coenzyme Q10
Health Food Raw Material Catalog- Spirulina
Health Food Raw Material Catalog-Broken Ganoderma lucidum spore powder
Health Food Raw Material Catalog-Melatonin
Health Food Raw Material Catalog - Fishoil
Imported health food record filling registration form
(If you need to obtain the English version of the above regulations or technical standards, or the official language version of your country, feel free to contact us)
Overseas health food filing:
1. For health food produced overseas that is a supplement of vitamins and minerals, please refer to the Guidelines for Raw Materials Available for the Filing of Health Food Produced Overseas (Applicable Filing)
2. Subject to excipients, dosage forms, health care functions and dosage.
3. The filing application company must be overseas, can be a brand owner or a production enterprise, but must have a production enterprise. That is, the filing approval field shows that the applicant unit is an overseas company, and the production unit is an overseas factory, or it can all be a production enterprise. (Fill in a responsible unit in China when applying)
4. Examination and approval by the State Administration for the Supervision and Administration of the Chinese Market.
Imported health food recordation process:
Provide samples for filing inspection--Organize filing materials--Apply for the username and password of the filing information system--Fill in the filing information system--Submit the filing materials--Review and obtain the filing certificate
Imported health food filing application materials (depending on the product):
1. Imported health food registration form, and the legal responsibility commitment letter of the filing person responsible for the authenticity of the submitted materials
2. A copy of the main registration certificate of the enterprise
3. Product formula material: product formula information
(The original registrant who has changed the formula needs to submit the adjusted formula and the description of the change of the formula)
4. Product production process materials, including production flowchart and descriptions
5. Safety and health function evaluation materials
5.1 Three batches of product efficacy ingredients or iconic ingredients, hygiene and stability inspection reports produced by the pilot scale process and above
(If the product formula of the original registrant has not changed, this item does not need to be provided)
5.2 Instructions on the rational use of raw materials and auxiliary materials, as well as instructions on label instructions and product technical requirements that comply with relevant regulations
6. Types, names and relevant standards of packaging materials
7. Sample draft of product label
8. Product technical requirements materials
9. A full-item inspection report issued by a legally qualified inspection agency that meets the technical requirements of the product
9.1 Qualification document of food inspection agency
9.2 Three batches of whole-item inspection reports that meet product technical requirements
10. Product name related search materials
11. Other materials indicating product safety and health functions
12. Qualification document issued by the competent government department or legal service agency of the country (region) where the product is produced, certifying that the filer is an overseas manufacturer of the listed health food
13. The certification document issued by the competent government department or legal service institution of the country (region) where the product is produced has been on the market for more than one year, or the safety report on the overseas sales of the product and the consumption of the population
14. The original text of technical regulations or standards related to health food in the country (region) of product production or international organization
15. The actual sample, Chinese translation and notarial certificate of the packaging, label, instruction manual listed in the country (region) where the product is produced
16. If the filing affairs are handled by the resident representative office of the overseas filing person in China, the "Registration Certificate of the Resident Representative Office of Foreign Enterprises in China" and its photocopy shall be submitted. If an overseas filing person entrusts a domestic agency to handle filing matters, it shall submit the original notarized power of attorney and a photocopy of the business license of the entrusted agency.
Registration of health food produced overseas:
1. For health food produced overseas (health food raw materials other than supplemented vitamins and minerals), please refer to the Guidelines for Raw Materials Available for Registration of Health Food Produced Abroad (Applicable Registration).
2. The company applying for registration must be overseas, can be a brand or a production enterprise, but must have a production enterprise. That is, the registration approval field shows that the applicant unit is an overseas company, and the production unit is an overseas factory, or it can all be a production enterprise. (Fill in a responsible unit in China when applying)
3. Examination and approval by the State Administration for the Supervision and Administration of the Chinese Market.
Application for health food registration requires a long time for professional review (usually 5-10 years), and has comprehensive requirements for product formula, preparation process, method demonstration, equivalence study, test report and clinical data, according to the specific situation of the product Contact us for the best registration solution.
Health food labels and instructions must comply with relevant national standards and requirements, and indicate the following:
(1) Health care function and suitable population;
(2) Edible methods and dosage;
(3) Storage method;
(4) The name and content of the functional ingredients;
(5) Health food approval number;
(6) Health food labels;
(7) Other label contents stipulated by relevant standards or requirements;
Additional instructions:
(1) The names of health food should be accurate and scientific, and should not use personal names, place names, code names and exaggerated names that are easy to misunderstand.
(2) The contents of labels, instructions and advertisements of health food must be true and meet the quality requirements of their products, and no propaganda suggesting that the disease can be cured.Health food produced in China for the record:
1. The "Health Food Raw Material Catalogue Nutrient Supplements" (that is, the vitamin and mineral catalog) and the five nutrients of coenzyme Q10, spirulina, melatonin, broken wall Ganoderma lucidum spore powder and fish oil can be recorded.
2. Subject to excipients, dosage forms, health care functions and dosage.
3. The filing application company must be a domestic production enterprise.
4. Provincial management and provincial approval, for example: Anhui production enterprise health food filing is approved by Anhui Provincial Market Supervision Administration.
Registration of health food produced in China:
1. Health food raw materials except vitamins, minerals, coenzyme Q10, spirulina, melatonin, broken wall Ganoderma lucidum spore powder and fish oil, please refer to the "Guidelines for Raw Materials Available for Registration of Health Food Produced in China (Applicable Registration)".
2. The company applying for registration must be Chinese, can be a brand or a production enterprise, but must have a production enterprise. That is, the registration approval field shows that the applicant unit is a Chinese company, and the production unit is a factory in China, or it can all be a production enterprise.
3. Examination and approval by the State Administration for the Supervision and Administration of the Chinese Market.
Test that may be involved in the filing or registration process:
(1) Functional ingredients or iconic ingredients, hygiene self-inspection;
(2) Functional ingredients or iconic ingredients, hygiene, and stability tests;
· Toxicology test;
· Animal testing;
· Human consumption evaluation test;
(3) Other tests (such as strain identification, strain virulence, stimulants, illegal drug test reports, etc.) are carried out when necessary;