- Fruit
- Edible Oil
- Stuffed Pasta
- Crops Grains Oilseeds and Rice
- Grain Milling and Malt
- Vegetable Dried Beans
- Natural Plant Spices
- Nuts and Seeds
- Dried Fruits
- Unroasted coffee&cocoa beans
- Special Dietary Foods
- Functional foods
- Eggs and egg products
- Meat and meat products
- Aquatic products
- Aquatic Animals
- Dairy
- Casings
- Bird's Nest
- Bee products
- GMO
- Non-edible animal products
- Chinese herbal medicine
- General Foods
- GACC Evaluates CA
- Cotton Supplier Registration
Special Dietary Foods
Categories of foods for special dietary purposes:
1. Formula foods for infants and young children
a. Formula foods for infants
b. Formula foods for older baby and young children
c. Formula infants foods for special medical purposes
2. Supplementary foods for infants and young children
a. Cereal supplementary food for infants and young children
b. Canned supplementary food for infants and young children
3. Food for Special Medical Purpose,FSMP (Food for Special Medical Purpose,FSMP(Excluding Infants Formula foods for special medical purposes)
4. Other foods for special dietary use other than the above categories (including nutritional supplements, sports nutrition foods, and other special dietary foods with corresponding national standards)
GACC registration conditions for overseas manufacturers of imported foods are as follows:
1. The food safety management system of the country/region where the manufacturer is located has passed GACC's equivalence assessment and/or review;
2. The manufacturer was established with approval by the competent authority of the country/region, and the manufacturer is under effective regulation by the competent authority;
3. The manufacturer has an established, effective food safety and sanitation management system and protection system, legally produces and exports food in the country/region, and ensures that foods exported to China comply with relevant Chinese laws, regulations, and national food safety standards;
4. Food exporting to China conforms with relevant inspection and quarantine requirements that have been agreed upon after discussion by the GACC and the competent authorities of the country/region.
• GACC registration process (text description)
• The flow chart for the GACC registration of imported food of overseas producers
• Registration Application of Overseas foods for special dietary purposes Imported Manufactures Recommended by Competent AuthorityThe General Administration of Chinese Customs, overseas official regulatory agencies and production enterprises will jointly promote:
-
01
Confirm the HS code and the applicable access method.
-
02
Obtain the account and password officially granted by the supervisor or account re-authentication.
-
03
Preparing documents in strict accordance with the Conditions and Key Points of Control Inspection for Registration of Overseas Production Enterprises of Imported foods for special dietary purposes and conduct self-assessment and fill in the registration application form.
-
04
Submit an electronic application, fill in the enterprise elements and upload relevant materials as required; if necessary, include but not limited to floor plan of factory and workshop, process flow chart, etc.
-
05
The competent authority reviews the summary and forwards the electronic application with other support materials required by GACC.
-
06
The GACC office will review whether the materials are complete and in compliance with the legal form, and decide whether to accept it or issue a correction notice to the competent authority.
-
07
The expert group evaluates and reviews, including a combination of document review and on-site review or other reasonable review methods, and forms a review report.
-
08
Review by the General Administration of Chinese Customs whether it meets the statutory requirements and standards.
-
09
GACC approve or disapprove the registration, and feedback the information to the competent authority and Enterprise (enterprise applicants can receive the registration feedback in Cifer system).
-
10
The enterprises approved for registration, China Customs will publish them online in due course.
Our assistance includes but is not limited to:
1. Assist in product classification, determine the corresponding registration method and the best implementation plan; confirm the applicable official competent authority approval number, enterprise type, precise customs code and inspection and quarantine code details.
2. Assist enterprises to obtain account numbers and passwords.
3. Assist enterprises to check the registration elements according to the "Conditions and Key Points of Control Inspection for Registration of Overseas Production Enterprises of Imported foods for special dietary purposes", complete the self-assessment, and provide continuous improvement plans based on the assessment results, so that they meet the registration conditions check points and China's national food production and product standards. Assist in sorting, screening, editing, and translating the required materials, so that the application materials meet the integrity, authenticity and validity.
4. Assist in filling out the "Application for Registration of Overseas Competent Authority Recommendation for Manufacturers of Imported Foods", submit an electronic application and upload relevant certification materials.
5. Follow up the application submitted by the competent authority, the basic status report on animal and plant epidemics, veterinary hygiene, public health, plant protection, pesticide and veterinary drug residues related to the exporting country (region), the registration management of food production enterprises and the requirements of enterprise hygiene standards, etc. The laws, regulations and standards of the exporting country (region), the organization and personnel of the competent authority of the exporting country (region), the quarantine of the exporting country (region) competent authority of its recommended enterprises, the assessment answer sheet of the actual situation of sanitation control, the exporting country (regional) competent authority's assessment of the actual situation. A statement that the companies that are recommends comply with Chinese laws and regulations.
6. Materials correction, when the written materials do not meet the registration requirements, assist the enterprise to complete the interpretation, sorting, screening, editing, translation, calibration and other standards and laws and regulations of the required materials within the specified time.
7. Acceptance by Chinese Customs and follow-up and communication of technical review by expert group, preparation and coordination before on-site review or video review.
8. Assist in successful access and follow up on the use of registration data for customs clearance, and respond immediately if there are any problems.
9. According to the change of the enterprise, assist the change application.
10. Other enterprise access and follow-up related support.
The Chinese national standards and regulations that is required to comply with include but are not limited to:
1. National Food Safety Standard Formula Food For Infants GB10765-20212. National Food Safety Standard Formula Food For Older Babies GB10766-2021
3. National Food Safety Standard Formula Food For Young Children GB10767-2021
4. National Food Safety Standard Good Manufacturing Practice For Powdered Formula For Infants And Young Children GB 23790-2010
5. National Food Safety Standard General Standard for Infant Formula for Special Medical Purposes GB 25596-2010
6. National Food Safety Standard General Rules on Formula Food for Medical Use GB 29922-2013
7. National Food Safety Standard GMP of Formula Food for Medical Use GB 29923-2013
8. National Food Safety Standard Food Labeling of Prepackaged Special Diet GB 13432-2013
9. National Food Safety Standard General Standard for Sports Nutrition Food GB 24154-2015
10. National Food Safety Standard Multi-nutrient Supplementary Food for Pregnant and Lactating Women GB 31601-2015
11. National Food Safety Standard Complementary food supplements GB 22570-2014
12. National Food Safety Standard Hygienic specifications of cannery GB 8950-2016
13. National Food Safety Standard Good manufacturing practice for drink factory GB 12695-2016
14. National Standard for Food Safety Food Additive Use Standard GB 2760-2014
15. National Standard for Food Safety General Rules for Labeling of Prepackaged Foods GB 7718-2011
16. National Food Safety Standard Food Nutrition Enhancer Use Standard GB 14880-2012
17. National Food Safety Standard General Hygienic Specification for Food Production GB 14881-2013
(If you need to obtain the English version of the above regulations or technical standards, or the official language version of your country, feel free to contact us)
Regulatory Measures
1. Overseas manufacturing enterprises exporting foods for special dietary uses to China may be recommended and registered by the competent authorities of the country (region) where they are located to the GACC, and can export their products to China after passing the registration procedures of the GACC.
2. Overseas exporters or agents and domestic importers shall record their information through the record-keeping system for importers and exporters of imported food and cosmetics of the General Administration of Customs of China.
3. Formula Registration Certificate of Infant Formula Milk Powder (Import)
4. Registration Certificate of Formula Food for Special Medical Purposes (Import)
5. Chinese labels of imported foods for special dietary uses shall be printed on the minimum sales package and no additional labels shall be added. The registration number of the overseas manufacturer in China or the registration number approved by the competent authority of the country (region) where the manufacturer is located shall be marked on the inner and outer packaging.
Solutions for Meeting the Regulatory Measures:
1. Solution: Overseas manufacturing enterprises shall fill out the application form through the CIFER registration system after the official recommendation, and the applicant enterprise shall meet the standards and requirements stipulated in the Compliance Checklist for Registration of Overseas Manufacturers of Imported Foods for Special Dietary Uses; After the GACC receives the application, the expert panel shall evaluate and examine the applicant through written inspection, video inspection, on-site inspection and other forms of inspection or any combination of the above, and register the overseas manufacturers of imported food that meet the requirements and give them the registration number in China.
2. Overseas exporters or agents and domestic importers shall record their information through the record-keeping system for importers and exporters of imported food and cosmetics of the General Administration of Customs of China.
Solution: Submit the application through “Internet + Customs” - “Record System for Importers and Exporters of Imported Food”.
3. Formula Registration Certificate of Infant Formula Milk Powder (Import)
Solution: Declare to the Center for Food Evaluation of the State Administration for Market Regulation according to the Administrative Measures for Product Formula Registration of Infants and Young Children Formula Milk Powder and other relevant laws and regulations.
4. Registration Certificate of Formula Food for Special Medical Purposes (Import)
Solution: Declare to the Center for Food Evaluation of the State Administration for Market Regulation according to the Administrative Measures for Registration of Formula Food for Special Medical Purposes and other relevant laws and regulations.
Information required for Customs declaration:
1. Certificate of Origin
2. Sanitary certificate (Certificate of free sale or health certificate)
3. Invoice, contract, packing list, Bill of Lading
4. Test report
5. List of ingredients
6. Certificate of Formula Registration of Formula Milk Powder for Infants and Young Children (Import) (only applicable to Formula Milk Powder for Infants and Young Children)
7. Registration Certificate of Formula Food for Special Medical Purposes (Import) (Only applicable to Formula Food for Special Medical Purposes)
8. Chinese label, label of foreign languages, and translation
9. Other materials or reports to be submitted
The following imported foods for special dietary purposes are monitored by Decree 248:
| HS Codes | Prodcut Name | CIQ Codes | Inspection and quarantine name | Product Category |
| 1901101010 | Premature infant/low birth weight infant formula (milk-based), breast milk nutritional supplement (milk-based) special infant formula (made from dairy products with total skimmed cocoa content <6% by weight) | 102 | Premature infant/low birth weight infant formula (milk-based), breast milk nutritional supplements (milk-based) special infant formula foods (total skimmed cocoa content by weight <6% dairy products) (breast milk nutritional supplements (milk-based) special Infant formula food) | Formula foods for special medical purposes |
| 1901101010 | Premature infant/low birth weight infant formula (milk-based), breast milk nutritional supplement (milk-based) special infant formula (made from dairy products with total skimmed cocoa content <6% by weight) | 101 | Preterm infants/low birth weight infant formula (milk-based), breast milk nutritional supplements (milk-based) special infant formula food (by weight total skimmed cocoa content <6% dairy products) (premature infant/low birth weight infant formula ( Milk-based) food) | Formula foods for special medical purposes |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 105 | Other retail packaged foods for infants and young children (by weight fully skimmed cocoa content <40% powder, starch or refined wheat; fully skimmed cocoa content by weight <5% dairy products) (bean-based older infants and young children formula food ) | Soy-based infant formula food |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 106 | Other retail packaged foods for infants and young children (by weight fully skimmed cocoa content <40% powder, starch or refined wheat; fully skimmed cocoa content <5% by weight dairy products) (cereal supplementary food for infants and young children) | Supplementary foods for infants and young children |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 107 | Other retail packaged foods for infants (canned supplementary foods for infants and young children (canned supplementary foods for infants and young children) | Supplementary foods for infants and young children |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 113 | Foods such as malt extract and grain flour and dairy foods (flour, starch, and wheat refined with skimmed cocoa content <40% by weight; dairy products with skimmed cocoa content <5% by weight) (cereal supplementary food for infants and young children) | Supplementary foods for infants and young children |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 104 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% powder, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) (bean-based infant formula food) | Soy-based infant formula food |
| 1901109000 | Other retail packaged foods for infants and young children (by weight of fully skimmed cocoa content <40% of flour, starch or refined wheat; by weight of fully skimmed cocoa content <5% of dairy products) | 114 | Malt Extract, grain and dairy food-food powder (by weight of the fully defatted cocoa <40% flour, starch, wheat purified; by weight fully defatted cocoa content of <5% milk, Ltd.) (Canned supplementary foods infant ) | Supplementary foods for infants and young children |
| 1901900000 | Foods and dairy foods such as malt extract and grain powder (by weight of fully skimmed cocoa content <40% of flour, starch, and wheat refined; by weight of fully skimmed cocoa content <5% of dairy products) | 115 | Foods such as malt extract and grain powder and dairy foods (prepared from flour, starch, and wheat with a content of skimmed cocoa <40% by weight; prepared from dairy products with a content of skimmed cocoa <5% by weight) (formula foods for special medical purposes) | Formula foods for special medical purposes |
| 2106100000 | Concentrated protein and artificial protein material | 106 | Concentrated protein and artificial protein substances (formula foods for special medical purposes) | Formula foods for special medical purposes |
| 2106909001 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula food | 103 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula food (amino acid metabolism disorder formula special medical purpose infant formula food) | Formula foods for special medical purposes |
| 2106909001 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula food | 106 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula (milk protein deep hydrolysis formula special medical purpose infant formula) | Formula foods for special medical purposes |
| 2106909001 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula food | 104 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula (lactose-free formula for special medical purpose infant formula) | Formula foods for special medical purposes |
| 2106909001 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special infant formula food | 105 | Lactose-free formula or low lactose formula, milk protein partially hydrolyzed formula, milk protein deep hydrolyzed formula or amino acid formula, preterm/low birth weight infant formula (non-dairy-based), amino acid metabolism disorder formula, breast milk nutritional supplement (non-dairy-based) Special formula for infants and young children (partially hydrolyzed milk protein formula for special medical purpose infant formula) | Formula foods for special medical purposes |
| 2106909019 | Other numbered unlisted foods containing endangered animal and plant ingredients | 111 | Other numbered unlisted foods containing endangered animal and plant ingredients (formula foods for special medical purposes) | Formula foods for special medical purposes |
| 2106909019 | Other numbered unlisted foods containing endangered animal and plant ingredients | 110 | Foods not listed in other numbers containing endangered animal and plant ingredients (canned supplementary foods for infants and young children) | Supplementary foods for infants and young children |
| 2106909019 | Other numbered unlisted foods containing endangered animal and plant ingredients | 109 | Foods containing endangered animal and plant ingredients that are not listed in other numbers (cereal supplementary foods for infants and young children) | Supplementary foods for infants and young children |
| 2106909090 | Food not listed in other numbers | 144 | Other foods not listed in the number (formula foods for special medical purposes) | Formula foods for special medical purposes |
| 2106909090 | Food not listed in other numbers | 141 | Other foods not listed in the number (breast milk nutritional supplements) | Formula foods for special medical purposes |
| 2106909090 | Food not listed in other numbers | 143 | Other foods not listed in the number (canned supplementary foods for infants and young children) | Supplementary foods for infants and young children |
| 2106909090 | Food not listed in other numbers | 142 | Other foods not listed in the number (cereal supplementary foods for infants and young children) | Supplementary foods for infants and young children |
| 2106909090 | Food not listed in other numbers | 151 | Other foods not listed in the number (maternal nutritional supplements) | Others (complementary food supplements, sports nutrition foods, etc.) |
| 2106909090 | Food not listed in other numbers | 150 | Foods not listed in other numbers (sports nutrition foods) | Others (complementary food supplements, sports nutrition foods, etc.) |
| 2106909090 | Food not listed in other numbers | 152 | Other foods not listed in the number (complementary food supplements) | Others (complementary food supplements, sports nutrition foods, etc.) |
1. What are the common mistakes in registration?
(1) The product was classified incorrectly, and the wrong access method and procedure were applied.
(2) Failure to strictly follow the "Conditions and Key Points of Control Inspection for Registration of Overseas Production Enterprises of Imported foods for special dietary purposes" to inspect the elements of the enterprise and conduct feasibility assessments.
(3) Electronic application has not been submitted. "Registration Application of Overseas foods for special dietary purposes Imported Manufactures Recommended by Competent Authority"
(4) Registration application materials or attachments submitted are not in English or Chinese.
(5) The file format is incorrect and cannot be opened.
(6) There is a vacancy in the application materials where the signature and seal of the competent authority is required.
(7) The necessary parts of the application form are incomplete.
(8) The attachments of the application form are incomplete or do not correspond to the description of the content of the application form, which cannot prove the validity of the content of the application form.
(9) There are descriptions in the application form and attached materials that are obviously inconsistent with Chinese regulations.
(10) The information of the exported products cannot be accurately listed in the application, so that the customs clearance failure and needs to be urgently added. (Overseas manufacturers generally register according to the actual product strictly corresponding to the HS CODE, but Chinese brokers or freight agencies generally classify HS CODE according to the most preferential tax rate or the lowest supervision conditions or the simplest operation methods. There are cases that different ports and different freight agencies use different HS CODE for the same foods. There is a possibility of different classification, and our agency have to deal with many emergencies caused by the reasonable but misplaced demands of different market entities.)
(11) The application materials provided are contradictory.
(12) The country/origin where the overseas producers is located export firstly some foods has not yet conducted conformity equivalent assessment of the GACC official regulatory system.
2. An example of GACC does not approve review feedback?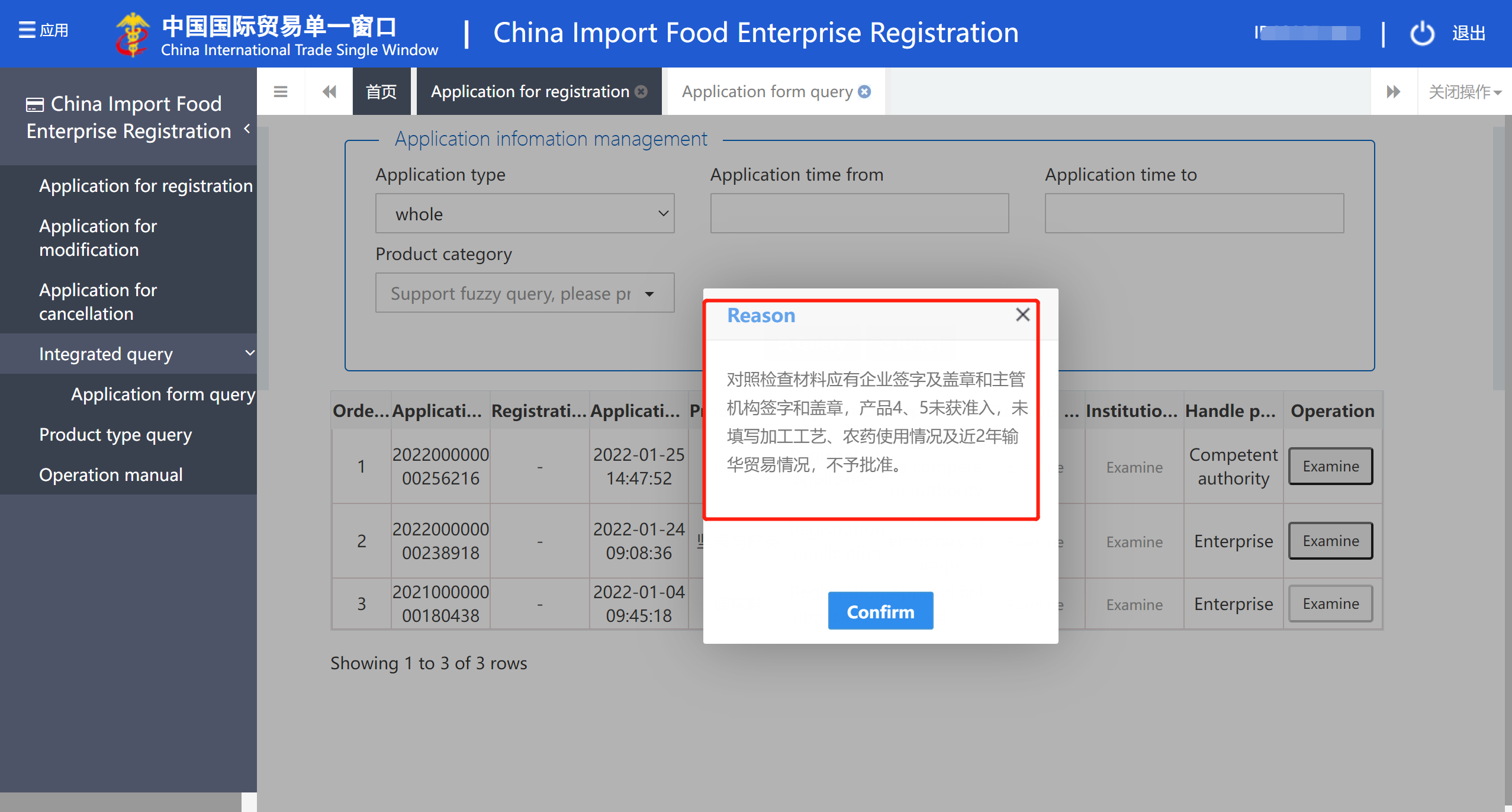
3. What's the labeling requirements of imported foods for special dietary purposes?
Answer: According to Decree No.249 Article 30:The packaging, labels, and marks of import food shall comply with the laws, regulations, national food safety standards of China, and shall be accompanied by an instruction manual in Chinese if the law so requires.The label of an imported dietary supplement or food for special dietary use in Chinese must be printed on the smallest sales package, and shall not be affixed thereto.
4. Can formula foods for special medical purposes be traded online?
Answer: According to the "Market Access Negative List (2018 Edition)", the prohibition of access is stipulated: the specific nutritional formula food in the formula food for special dietary purpose shall not be traded online.
5. What are the changes between food for special dietary purposes application and previous customs record filling for overseas manufacturers?
Answer: Before enforcement of Decree 249, only imported milk powder and FSMP overseas manufacturers are required to make application, now it extends to the categories like formula foods for infants and young children, supplementary foods for infants and young children and sports nutrition foods.
6. Do exporters or shippers need to record?
A: Yes, the exporter of the trade contract or shipper of BL has to do ire (Original AQSIQ) record. After recording will get an 11-digit record number, the record does not issue certificate nor validity limit. It should be noted that this record is very easy, we are obliged to help applicant record free of charge. Watch out for fraudulent websites.
(In China's administrative reform, AQSIQ no longer exist)
The Chinese national standards and regulations that is required to comply with include but are not limited to:
1. National Food Safety Standard Formula Food For Infants GB10765-20212. National Food Safety Standard Formula Food For Older Babies GB10766-2021
3. National Food Safety Standard Formula Food For Young Children GB10767-2021
4. National Food Safety Standard General Standard for Infant Formula for Special Medical Purposes GB 25596-2010
5. National Food Safety Standard General Rules on Formula Food for Medical Use GB 29922-2013
6. National food safety standards-Labeling of prepackaged foods for special dietary use GB 13432-2013
7. National Food Safety Standard General Standard for Sports Nutrition Food GB 24154-2015
8. National Food Safety Standard Multi-nutrient Supplementary Food for Pregnant and Lactating Women GB 31601-2015
9. National Food Safety Standard Complementary food supplements GB 22570-2014
10. National Standard for Food Safety General Rules for Labeling of Prepackaged Foods GB 7718-2011
12. National Standard for Food Safety Food Additive Use Standard GB 2760-2014
13. National Food Safety Standard Food Nutrition Enhancer Use Standard GB 14880-2012
(If you need to obtain the English version of the above regulations or technical standards, or the official language version of your country, feel free to contact us)
Imported Food Compliance Services
2. Regulatory requirements and compliance procedures related to food import
3. Special product pre-license consultation
4. Consultation on other food import problems
Product technical compliance support
Purpose: To determine whether food can be imported into China
1. Determine the product classification and whether it can be imported
2. Check whether the product is illegally added or the dosage and content are not compliant
3. Whether the imported food meets the national food safety standards
4. If there is any non-compliance, propose amendments
Product Label Review
Purpose: To ensure that the Chinese labels of imported products meet the requirements
1. Review the original product standard and give suggestions for revision
2. Review Chinese labels, translate and make Chinese labels
Food for Special Medical Purpose Registration Inquiry
2. FSMP Registration Documents Review( Formula,Label, Raw materials and research consulting)
3. FSMP Registration Application
New food additives Compliance Application
2. New categories of food additive identification
3. Food additive scope of application or usage expansion
4. New categories of food additive compliance application
New food raw material declaration and consultation
1. New food raw material compliance consultation and training
2. Identification of new food raw materials
3. Substantial Equivalence Argument
4. New food raw material declaration